Utilizing Dipicolinic Acid as Key Intermediates for the Synthesis of Pyridine
Pyridine stands as a fundamental nitrogen-containing heterocycle in organic chemistry, crucial for producing various pharmaceuticals, agrochemicals, and dyes. Among its notable derivatives, Dipicolinic Acid (DPA) emerges as a critical intermediate for synthesizing a wide array of organic compounds. Recognized for its solubility in both water and organic solvents, DPA is a colorless solid powder with the formula C7H5NO4. UniVOOK Chemical distinguishes itself by offering globally high-purity DPA, characterized by exceptionally low levels of heavy metals and impurities. This quality assurance has garnered endorsements from three leading international companies within the food, animal nutrition, and feed additives sectors, highlighting DPA’s remarkable quality and versatility.
Synthesizing Pyridine through Dipicolinic Acid
Acquiring Dipicolinic Acid
Dipicolinic Acid or Pyridine-2,6-dicarboxylic acid serves as a crucial pharmaceutical synthesis intermediary with wide-ranging applications. Its natural occurrence in bacterial spores is limited, making extraction impractical for industrial needs. The synthesis journey began in 1935 with Professors ALVIN.W.Singer and S.M.MCELVAIN at the University of Wisconsin, who achieved a 64% yield by oxidizing 2,6-dimethylpyridine with potassium permanganate in water. Advancing this method, UniVOOK Chemical now optimizes the oxidation of 2,6-lutidine, significantly enhancing yield to 98%. Through our unique purification process, we ultimately provide our customers with products of 99.0-99.5% purity.

The critical role of Dipicolinic Acid’s purity cannot be overstated, as it directly influences the efficiency and success of further synthesis processes. High purity levels are essential to minimize side reactions and ensure optimal product yields, especially vital in pharmaceutical applications where impurities can compromise the safety and effectiveness of the final product.
Transforming Dipicolinic Acid into Pyridine
The journey from Dipicolinic Acid to Pyridine primarily involves decarboxylation, stripping away the two carboxyl groups. This transformation may entail various steps, from activating the acid groups to their elimination, culminating in pyridine formation.
The process requires specific catalytic systems or reagents to facilitate decarboxylation efficiently. Utilizing catalysts like metal oxides or complexes can significantly enhance conversion efficiency, minimizing energy requirements and accelerating reaction rates.
Optimizing reaction conditions—temperature, pressure, and catalyst concentration—is pivotal for efficient conversion. Such controlled conditions not only boost Pyridine yield but also improve reaction selectivity, effectively reducing by-products.
Finally, yield and selectivity are paramount in this conversion process. The objective is to maximize Pyridine production while curtailing unwanted side products, a balance achieved through strategic catalyst selection, reaction condition optimization, and meticulous purification methods to isolate and retrieve high-quality Pyridine.
Utilization of Pyridine from Dipicolinic Acid Across Industries
In Pharmaceuticals
- Pyridine Derivatives for Drug Development
The pharmaceutical sector greatly benefits from pyridine derivatives, leveraging their structural flexibility to create a plethora of active pharmaceutical ingredients (APIs) and intermediates. This versatility enhances the drugs’ effectiveness and their absorption, distribution, metabolism, and excretion (ADME) properties.
- Pharmaceutical Innovations with Pyridine
Pyridine is integral to crafting key pharmaceuticals, including sulfapyridine for anti-inflammatory purposes, pindolol for hypertension, and the antibiotic rifampicin. These derivatives are essential in the battle against a wide array of health conditions.
In Agrochemicals
- Enhancing Pesticides and Herbicides with Pyridine
In agriculture, pyridine-based compounds are crucial for formulating pesticides and herbicides, significantly aiding in pest and weed management. This support is vital for safeguarding crops and ensuring enhanced agricultural yields.
- The Benefits of Pyridine in Agriculture
Pyridine derivatives are favored in agrochemicals for their ability to be tailored for specific actions, offering precise effectiveness and minimal environmental footprint. Their application is key to developing safer agrochemicals and promoting sustainable farming methods.
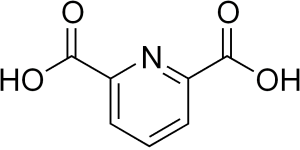
In Material Science
- Pyridine in Polymer Engineering
Material science harnesses pyridine derivatives as essential elements in polymer creation. They bring specific functionalities and modify polymers’ physical attributes, making them applicable in diverse fields, from protective coatings to high-performance materials.
- Characteristics of Pyridine-based Polymers
Polymers incorporating pyridine units stand out for their thermal resistance, conductive properties, and resilience against chemicals. Such polymers find use in cutting-edge sectors, including electronics, automotive, and healthcare products.
Versatile Applications and Intermediates
Dipicolinic Acid serves as a foundational ingredient in the synthesis of various pyridine-based products. Its utility spans beyond pharmaceutical intermediates to include roles as an enzyme inhibitor in research and healthcare. Additionally, its derivatives are instrumental in animal nutrition, evidencing the wide-reaching applications of Dipicolinic Acid in enhancing industry standards and product efficacy.
Challenges and Future Directions in Pyridine Synthesis from Dipicolinic Acid
Challenges in Synthesis
- Optimizing Reaction Conditions
Achieving high yield and selectivity in the synthesis of Pyridine from Dipicolinic Acid presents a significant challenge. Optimizing reaction parameters—such as temperature, pressure, and catalyst concentration—is crucial for enhancing the efficiency of the process and the purity of the final product. This optimization requires a deep understanding of the reaction mechanism and the interplay between various factors.
- Developing Sustainable Synthesis Methods
Another challenge lies in transitioning to greener and more sustainable synthetic routes. The conventional synthesis of Pyridine often involves harsh chemicals and conditions, leading to environmental concerns. There is a growing demand for methods that reduce waste, lower energy consumption, and use renewable resources or less harmful reagents.
Future Prospects and Innovations
- Innovative Catalytic Systems
The exploration of novel catalytic systems stands as a promising avenue for future research. Innovations in catalysis could lead to more efficient, selective, and eco-friendly processes for Pyridine synthesis from Dipicolinic Acid. Metal-organic frameworks (MOFs), enzymes, and nanoparticle-based catalysts are among the potential candidates for enhancing reaction rates and yields while minimizing environmental impact.
- Expanding Applications in New Fields
The integration of Pyridine derivatives into emerging technologies and fields offers exciting prospects. In nanotechnology, for instance, Pyridine-based molecules can be utilized to design functional nanomaterials for drug delivery, sensing, and electronics. The unique properties of Pyridine units, such as their ability to form stable complexes with metals and conduct electricity, make them valuable components in the development of nanoscale devices and materials.
The journey of Pyridine synthesis from Dipicolinic Acid is marked by both challenges and opportunities. Addressing the current obstacles through innovative research and technology could not only improve the synthesis process but also unlock new applications of Pyridine derivatives, thereby contributing to advances in pharmaceuticals, agriculture, material science, and beyond.
In Closing
Pyridine synthesis, utilizing Dipicolinic Acid as a key intermediate, represents a pivotal advancement in the field of organic chemistry, offering a gateway to a multitude of applications in pharmaceuticals, agrochemicals, and material science. This approach not only underscores the versatility and potential of Dipicolinic Acid in synthesizing valuable compounds but also highlights the ongoing quest for optimization and sustainability in chemical processes. As researchers continue to explore innovative catalytic systems and greener methodologies, the synthesis of Pyridine from Dipicolinic Acid stands at the forefront of scientific exploration, promising new avenues for advancements and applications across diverse industries.
Access Our Product Catalog and More to Discover High-Performance Chemicals Tailored to Your Business Needs