4-Bromo-2, 6-difluoroaniline for Pharmaceuticals
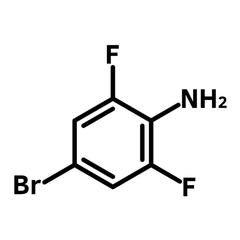
4-Bromo-2,6-difluoroaniline is a valuable aniline derivative with the CAS number 67567-26-4. With a molecular weight of 208.01 g/mol, it is characterized by a white crystalline solid form. The structure features a bromine atom in the para-position and two fluorine atoms in the ortho-positions on the benzene ring, leading to varied chemical reactivity. This diversity makes it a versatile building block in chemical synthesis. Its bromine moiety is pivotal for Pd-catalyzed coupling reactions, enhancing molecular complexity.
The amine group serves as a nucleophile in various reactions and is a precursor for azo compounds when oxidized. Additionally, the fluorine atoms modulate molecular orbital energy levels, facilitating the design of macromolecules through nucleophilic aromatic substitutions. Given these properties, 4-bromo-2,6-difluoroaniline is of significant interest in pharmaceutical research and development, where its application can lead to innovative therapeutic agents. UniVOOK Chemical, established in 2008 and headquartered in Shanghai, China, is a key supplier of this compound, offering custom manufacturing services worldwide.
The Chemical Essence of 4-Bromo-2,6-difluoroaniline
Molecular Structure Unveiled
The core of 4-Bromo-2,6-difluoroaniline is marked by its aromatic nature, decorated with a bromine atom and two fluorine atoms strategically positioned around an aniline moiety. This unique molecular arrangement not only delineates its chemical identity but also shapes its diverse functional applications.
Examining Physical Characteristics
lTransition Points: Manifesting as a crystalline entity, its melting and boiling points underscore its purity and inherent stability.
lSolubility Insights: Its solubility profile shows a preference for organic solvents such as DMF and DMSO, while its aromatic bonds limit water solubility.
lAppearance: The compound’s white crystalline form is indicative of the high purity typical for aniline derivatives.
Chemical Reactivity Explored
The reactive nature of 4-Bromo-2,6-difluoroaniline springs from its functional groups. The bromine atom facilitates essential coupling reactions, and the amine group actively engages in nucleophilic or oxidative transformations. The fluorine atoms, too, play a crucial role, enabling sophisticated molecular constructions through nucleophilic aromatic substitutions.
Stability Considerations
While generally stable under routine conditions, this compound watches cautiously against oxidizing environments. The incorporation of fluorine significantly elevates its thermal durability, endorsing its use in reactions demanding high temperatures.
UniVOOK Chemical’s Unique Offerings
lHarmonious Functional Groups: The compound’s array of functional groups renders it a versatile candidate for a multitude of synthesis processes.
lAdvancing Technology: Its role extends into the realm of semiconductors, solar panels, and OLED technology, attributed to fluorine’s distinctive electronic properties.
lAssurance of Purity: UniVOOK Chemical’s commitment to a minimum of 98% purity guarantees top-tier quality for all applications.
lGlobal Distribution Mastery: With a comprehensive logistics network, UniVOOK Chemical excels in delivering products promptly and efficiently to a worldwide clientele.
Pathways to the Synthesis of 4-Bromo-2,6-difluoroaniline
Synthesis Route Diversity
The synthesis of 4-Bromo-2,6-difluoroaniline traverses through various pathways, from straightforward halogenation to intricate organometallic procedures, each method bringing its own set of nuances.
Insight into Predominant Synthesis Techniques
lHalogenation Direct Approach: This method revolves around precise bromination techniques, demanding meticulous control over the reaction conditions for optimal selectivity.
lPalladium-Catalyzed Cross-Coupling Elegance: Through palladium catalysis, this technique adeptly introduces a bromine atom to a fluorinated aniline precursor, celebrated for its effectiveness in generating complex structures.
Analyzing Synthesis Methodologies
lHalogenation Strategy: Appreciated for its cost-effectiveness and simplicity, this approach may sometimes struggle with selectivity, potentially leading to unintended side products.
lPalladium-Catalyzed Approach: Distinguished for its precision and high yields, this method incurs additional costs due to the use of specialized catalysts and stringent reaction conditions.
Ultimately, the selection of a synthesis strategy is governed by the desired outcome, weighing factors such as purity, yield, and the logistical aspects of material and equipment availability.
Leveraging 4-Bromo-2,6-difluoroaniline in Pharmaceutical Development
Integral to Drug Innovation
4-Bromo-2,6-difluoroaniline stands at the forefront of pharmaceutical advancement, heralded for its ability to underpin the synthesis of diverse therapeutic agents. Its distinct structural characteristics enable a vast array of chemical transformations, fostering the development of groundbreaking drugs. These drugs not only exhibit superior efficacy and specificity but also boast optimized pharmacokinetic attributes, making this compound indispensable in pushing the boundaries of medicinal chemistry.
In the Heart of Drug Formulations
Though the specific drugs utilizing 4-Bromo-2,6-difluoroaniline remain under wraps due to proprietary rights, it’s known to play a crucial role across a spectrum of therapeutic areas. From oncology and cardiology to neurology, it aids in crafting treatments like kinase inhibitors, antiviral therapies, and anti-inflammatory agents, demonstrating its extensive utility in pharmaceutical sciences.
Diverse Mechanisms for Disease Intervention
The mechanism by which 4-Bromo-2,6-difluoroaniline-based drugs exert their effects is as varied as the diseases they target. In cancer treatment, for instance, they may thwart vital enzymes or disrupt signaling pathways essential for tumor growth, while in other diseases, they might influence the behavior of specific receptors or ion channels, showcasing the compound’s flexibility in therapeutic
applications.
Prioritizing Safety in Use
Managing Toxicity Risks
4-Bromo-2,6-difluoroaniline shares the complexity of many synthetic compounds used in drug production, presenting potential toxicity that demands careful oversight. Its possible cytotoxic and genotoxic impacts necessitate thorough risk assessments to ensure that its pharmaceutical applications are safely realized.
Ensuring Safe Handling Practices
The safe management of 4-Bromo-2,6-difluoroaniline involves stringent safety protocols, including the adoption of personal protective equipment, ensuring proper ventilation or the use of fume hoods, and adhering to strict disposal procedures. Such measures are essential to mitigate exposure risks throughout its research, development, and manufacturing lifecycle.
Minimizing Environmental Footprints
The production and disposal of 4-Bromo-2,6-difluoroaniline are subject to ongoing evaluation to limit environmental repercussions. Adopting eco-friendly manufacturing techniques and disposal practices is crucial, with regulations in place to guide the preservation of environmental integrity.
Forward-Looking Research and Prospects
Innovations in Synthesis and Applications
The method of synthesizing 4-Bromo-2,6-difluoroaniline has seen remarkable improvements, with a focus on sustainable, efficient, and economically viable approaches. These include adopting green chemistry practices and employing novel catalysts that lessen the environmental impact of its production processes.
Trends Shaping Future Pharmaceuticals
The drive towards more targeted and personalized medical treatments positions 4-Bromo-2,6-difluoroaniline as a pivotal component. It supports the creation of therapies that not only enhance patient outcomes but also minimize adverse effects, aligning with the shift towards precision medicine.
Exploring New Horizons in Drug Development
The potential of 4-Bromo-2,6-difluoroaniline in the pharmaceutical industry remains vast, with opportunities in novel drug delivery mechanisms and addressing newly emerging health conditions. Ongoing efforts to refine synthesis methods and improve safety profiles promise to reinforce its utility in discovering and developing the next wave of pharmaceutical innovations.
Closing Thoughts
4-Bromo-2,6-difluoroaniline has emerged as a cornerstone in pharmaceutical development, offering a versatile platform for the creation of diverse therapeutic agents. Its unique structural properties enable the synthesis of drugs with enhanced efficacy, selectivity, and safety profiles, addressing a broad spectrum of diseases. Despite its toxicity and environmental concerns, advancements in safe handling and sustainable manufacturing practices are ensuring its responsible use. As research progresses, 4-Bromo-2,6-difluoroaniline continues to play a pivotal role in the evolution of targeted and personalized medicine, promising to significantly impact future healthcare innovations. Its enduring importance in drug development underscores the compound’s invaluable contribution to advancing medical science and improving patient outcomes.
Access Our Product Catalog and More to Discover High-Performance Chemicals Tailored to Your Business Needs